

Although many tests performed at the point-of-care are CLIA-waived, many nonwaived platforms are specifically designed for use outside a central laboratory. It is tempting to define POCT as laboratory tests that are CLIA-waived, but this distinction is too narrow. It is even conceivable, given the following considerations, that POCT could be performed in the central laboratory. Therefore, the location at which a laboratory test is performed does not classify it one way or the other. Their location near the patient does not influence the accreditation standards they have to meet.įor regulatory purposes, satellite laboratories are generally considered extensions of the central laboratory service, rather than a separate classification such as POCT. POCT is often regarded as tests performed outside of a central laboratory, but this definition also is unsatisfactory, as limited-service satellite laboratories staffed by laboratory personnel are considered clinical laboratories (or sometimes blood gas laboratories) but not POCT services, at least for accreditation and regulatory standards.
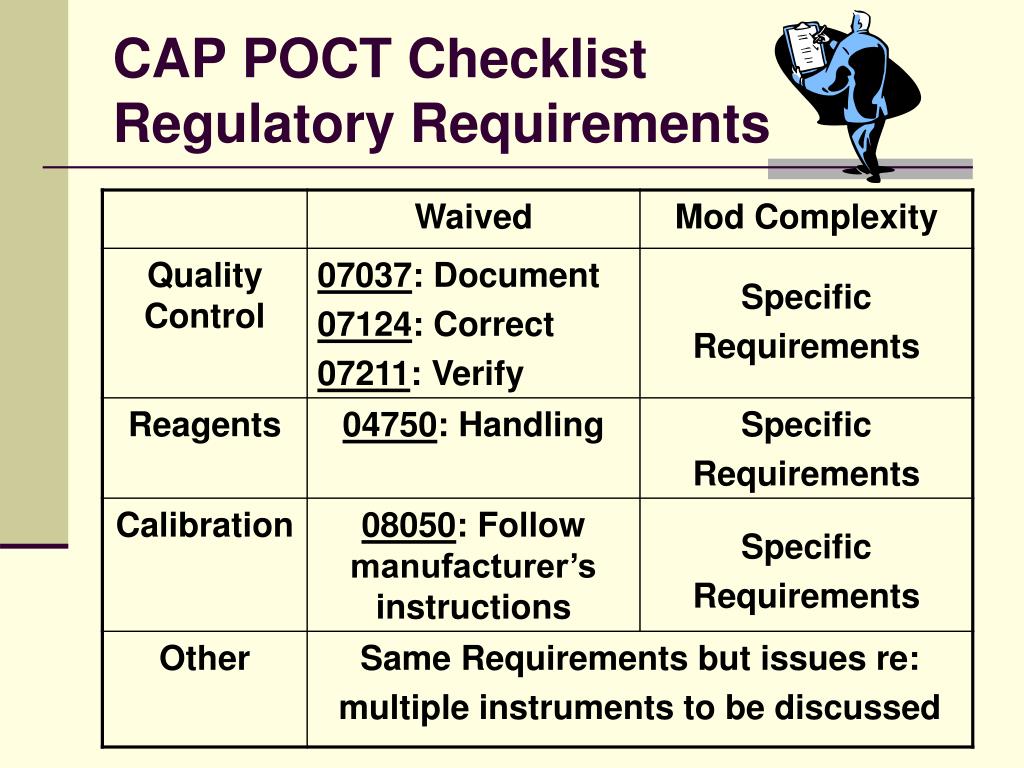
All were derived from the proximity of the laboratory test to the patient or central laboratory, but this distinction is relative and imprecise. There is some ambiguity with the term POCT and its predecessors, bedside testing, near patient testing, and less frequently, ancillary or decentralized testing. In this brief review, we will discuss some POCT-related regulatory issues in the hospital environment, and potential ways to satisfy those requirements. This is an unfamiliar and uncomfortable role for laboratory medicine professionals, who are highly trained to promote quality patient care and efficient use of resources. Consequently, nurses and other providers often see POCT coordinators as police who indiscriminately enforce regulations that seem onerous at best, and detrimental to patient care at worst. In addition, despite rapid growth of POCT methods and use, POCT operators often have limited understanding of the regulatory and accreditation requirements for licensure, training, procedures, and documentation. Modern POCT devices are greatly improved, but capturing the data required to document compliance remains a labor-intensive process. Today, point-of-care coordinators use a variety of processes to maintain control over multiple devices and monitor regulatory compliance of many operators at locations across healthcare enterprises.Įarly POCT methods were mostly manual, with minimal or no quality control and limited data management capabilities. Deploying POCT devices created challenges for laboratory management, especially in ensuring the proper use of these devices. Beginning in the 1980s, point-of-care testing (POCT) departed from conventional clinical laboratory medicine by decentralizing laboratory services.


 0 kommentar(er)
0 kommentar(er)
